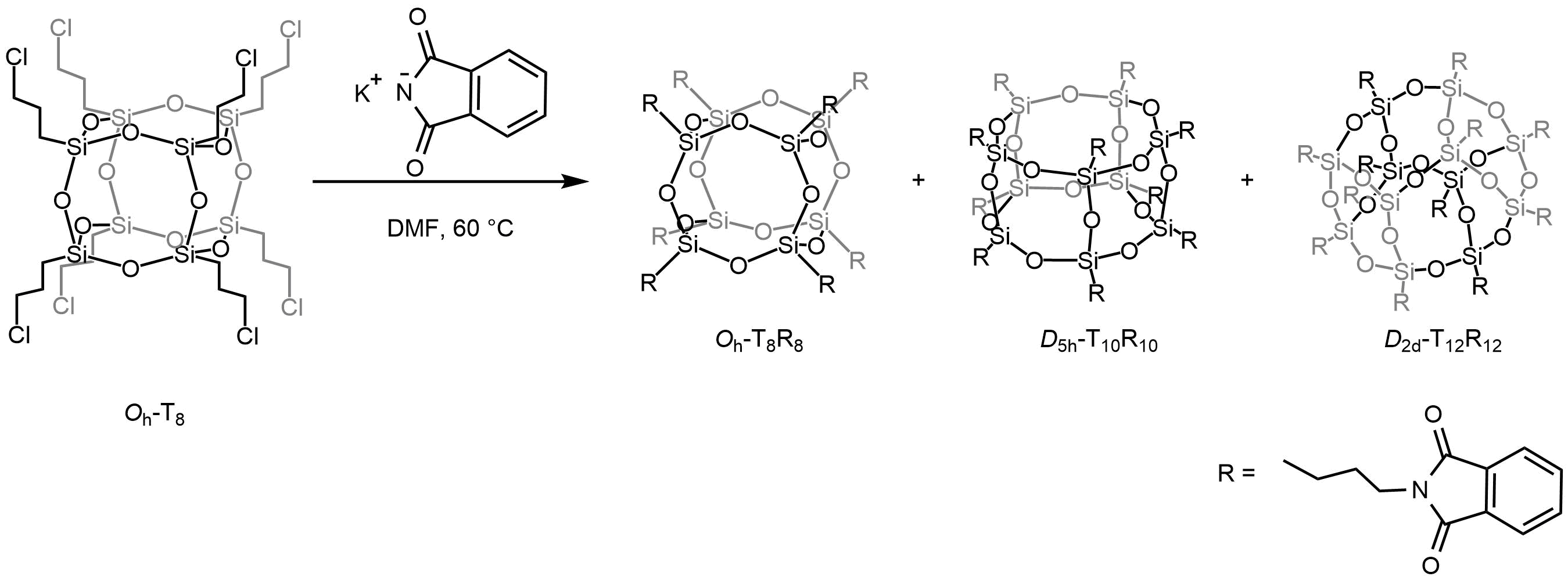
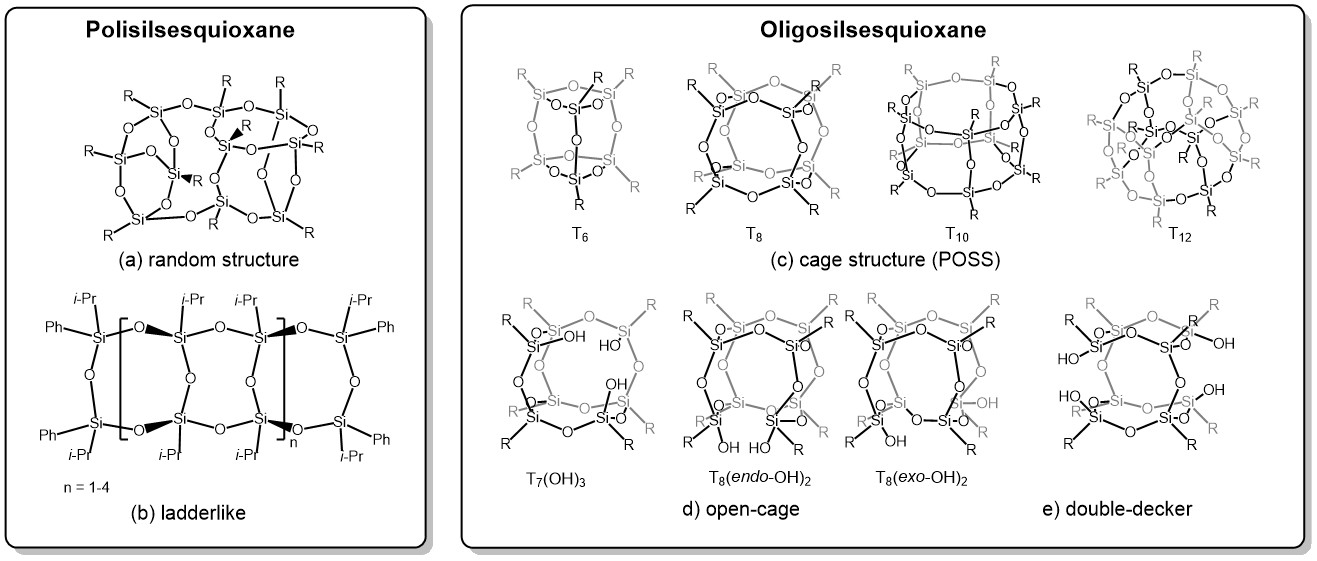
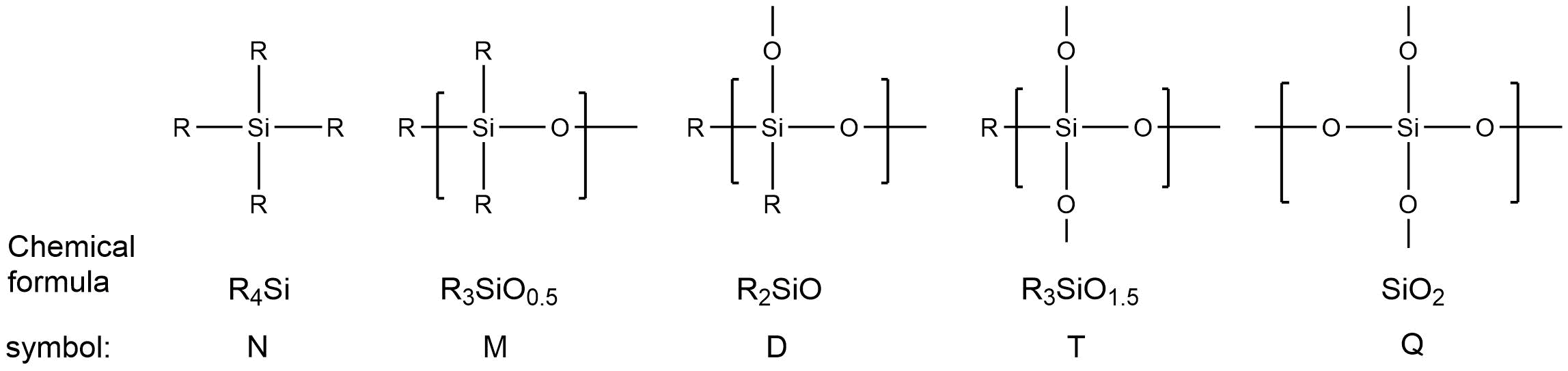
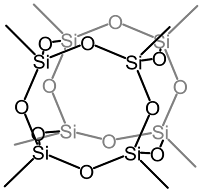
Octavinyloctasilsesquioxane (POSS-Octavinyl substituted) and its derivatives

Octavinyloctasilsesquioxane was first prepared in 1978 with a yield of <10% by K. Andrianov 1 and co-workers in the trichlorovinylsilane hydrolysis reaction. Since then, many groups have tried to improve the method of obtaining it. 2-4 In 1997, P. Harrison and Ch. Hall, when examining the hydrolysis of CH 2 =CHSiCl 3 in ethanol, they obtained (CH 2 =CHSiO 1.5 ) 8 with an efficiency of 30%. 5 The use of an ion exchange resin as a catalyst for the hydrolysis and condensation of CH 2 =CHSiCl 3 contributed to an increase in the reaction efficiency to 40%. 6 Compound (CH 2 =CHSiO 1.5 ) 8 was obtained with a yield of 80% using CH 2 =CHSi(OEt) 3 during hydrolysis and tetramethylammonium hydroxide as a phase transfer reagent. 7 Octavinyloctaasilsesquioxane derivatives can be obtained as a result of typical alkene reactions, such as: thiolation, phosphination, hydrosilylation, epoxidation, as shown in scheme below. Thiolation with reagents such as thiophenol or thiocyclohexane can b