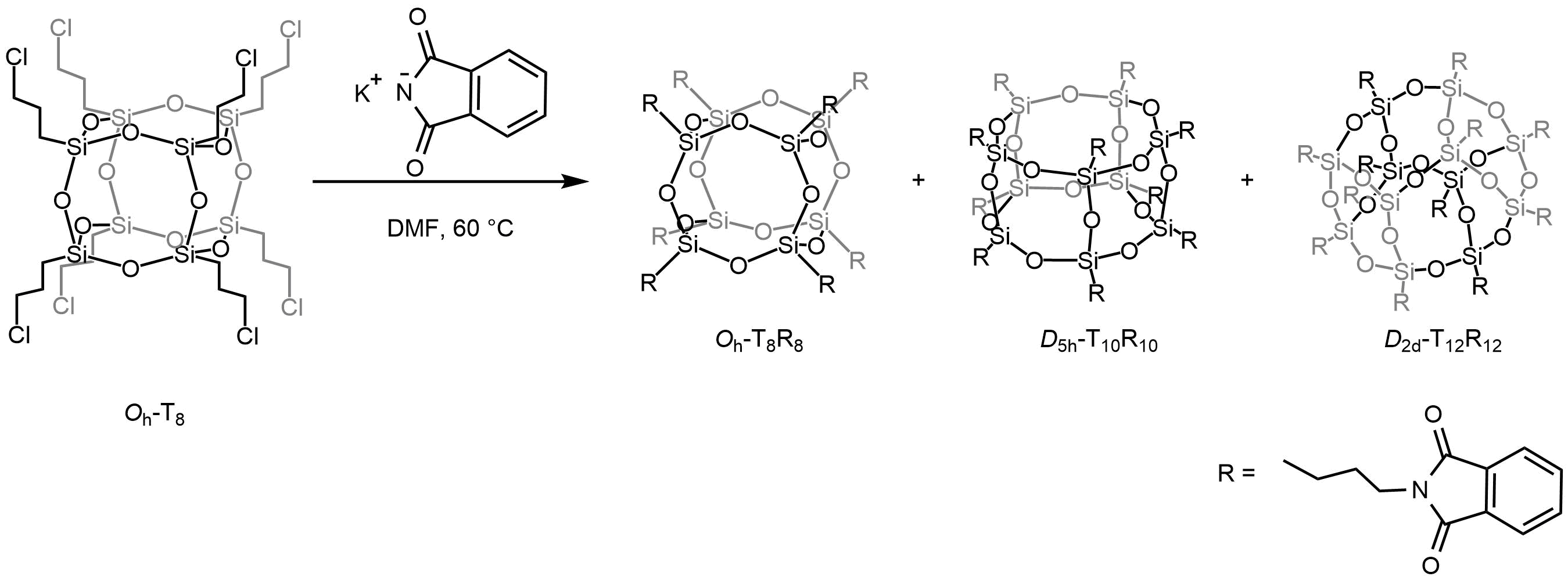
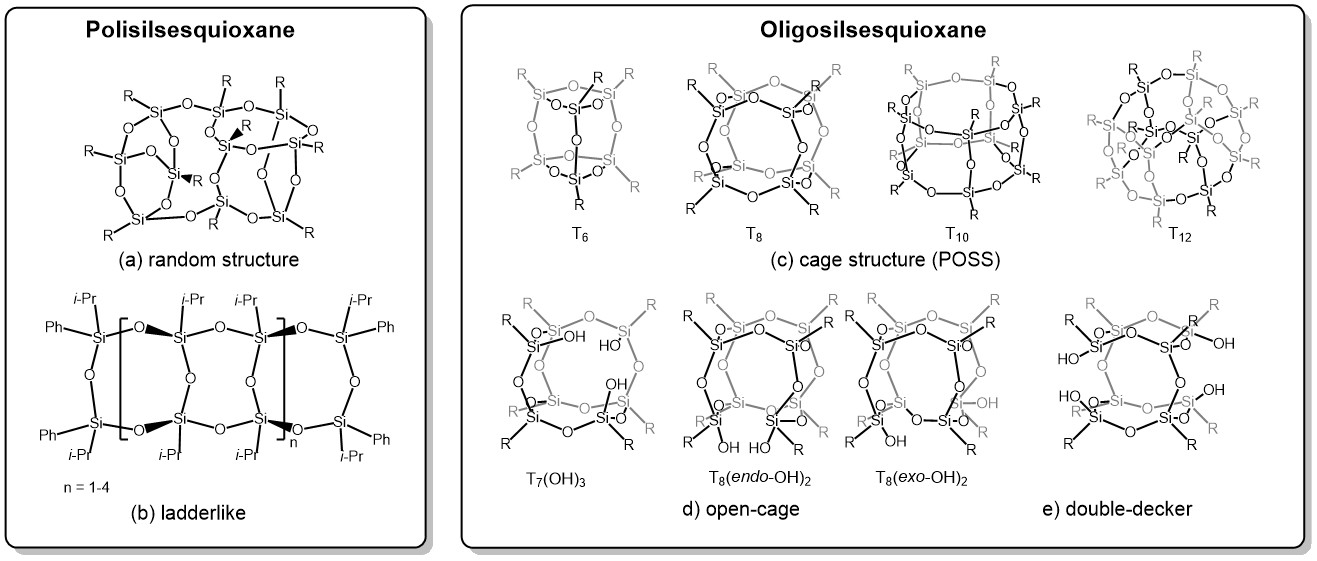
POSS as a photocatalyst

The publication titled "Polyhedral oligomeric silsesquioxane difluoroboron complexes as cooperative octo-site catalysts for the photooxidation of sulfides to sulfoxides" by Mateusz Janeta and Sławomir Szafert, published in Inorganic Chemistry Frontiers on April 17, 2025, presents a study on the development of novel metal-free photocatalysts. These catalysts are based on polyhedral oligomeric silsesquioxanes (POSS) functionalized with difluoroboron complexes. Scheme 1 Synthesis of POSS-sal-BF 2 , POSS-tert-BF 2 and POSS-npht-BF 2 . Isolated yields in parentheses. Key Highlights: Catalyst Design: The researchers synthesized three new difluoroboron-functionalized POSS complexes— POSS-tert-BF₂ , POSS-sal-BF₂ , and POSS-npht-BF₂ —derived from imine-functionalized POSSs. Photocatalytic Performance: These complexes demonstrated exceptional efficiency in the aerobic photooxidation of sulfides to sulfoxides, signific...