Decameric silsesquioxanes
There is little information available in the literature concerning pure, isolated larger cages like the aforementioned heptahedral T10. A small amount of T10 type POSS is usually formed as a by-product during the preparation of T8 type silsesquioxanes. For example, during the preparation of T8H8 by the hydrolytic condensation of HSiCl3, a small amount of T10H10.1 is formed in addition to the main product.
The formation of more thermodynamically unstable POSS of the T10 or even T12 type occurs primarily as a result of the reorganization of the core of the T8 cage. The transformation of the T8 cage into a larger one has been published by several research groups.2–4 For example, V. Ervithayasuporn observed the reorganization of octakis (3-chloropropyl) octasilsesquioxane by reaction with sodium methacrylate,5 sodium phenoxides,6 or potassium phthalimide.7 These reactions resulted in the formation of mixture of compounds of the type T8, T10 and T12 as shown in the scheme below.
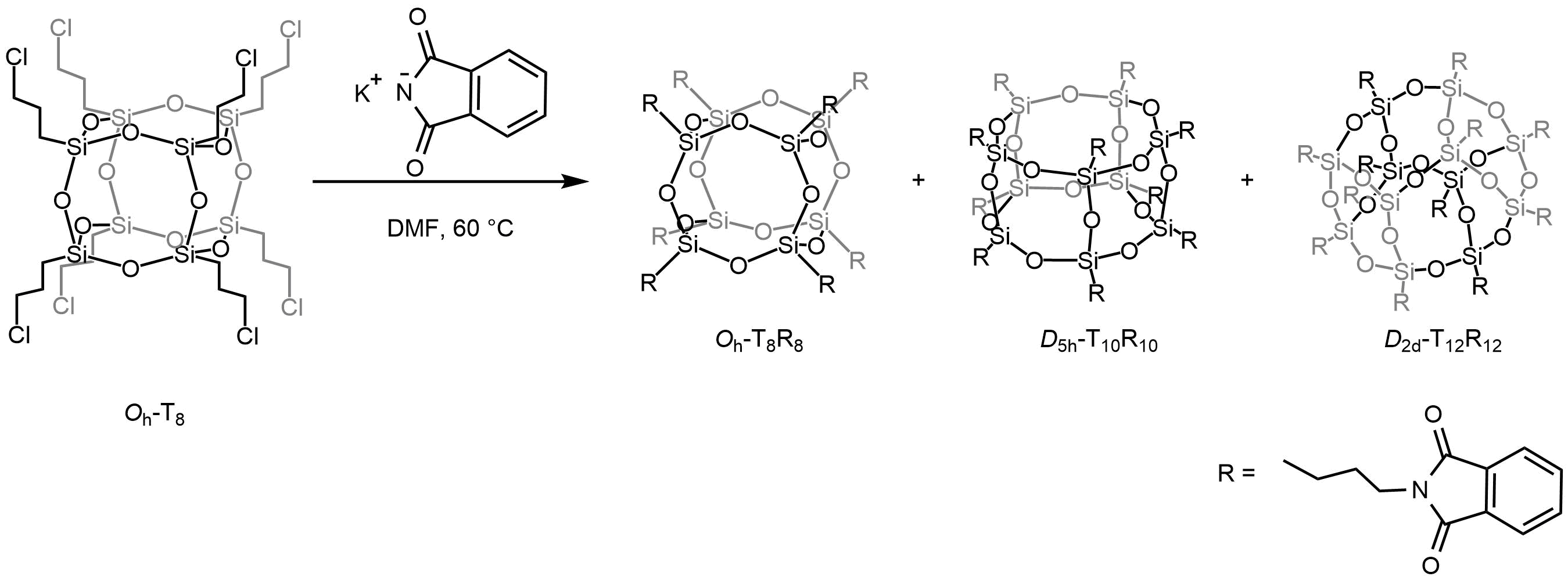
Reorganization of the POSS core during synthesis its phthalimide derivative
In addition to the above methods, a method of obtaining T10 cages by converting silsesquioxane with fluoride ions is known in the literature. E. Rikowski et al. Discovered that T10 and T12 type POSS can be formed by transforming a T8 cage containing a 3-chloropropyl moiety. The reaction takes place in acetonitrile using Na2SiF6 and 18-crown-6 as catalyst. The yield of this conversion is: 28% (T8), 61% (T10) and 11% (T12).8 The same authors8 also showed that T8[alkyl]8 analogs composed of cages containing 10 or 12 silicon atoms can be obtained. These compounds result from base catalyzed reorganization of the core. For example, T8[C2H5]8 when heated with K2CO3 in acetone forms a mixture of T10[C2H5]10 and T12[C2H5]12 with an efficiency of 55 and 4% respectively (41% remains unreacted). The remaining compounds of formula T8[alkyl]8 only form T10[alkyl]10 with low yield (less than 18%). Y. Kawakami et al. Described the formation of POSS T8, T10 and T12 during the hydrolysis of 4-substituted phenyltriethoxysilane in the presence of tetrabutylammonium fluoride. hexane or ethanol/hexane mixtures.
Reorganization of the siloxane cage-like core (T8 → T10) can be easily performed, including isolation of intermediates, and cage rearrangement achieved by using superacid CF3SO3H (TfOH). Moreover, T10-like SQs can be obtained in a one-step reaction by alkoxysilane condensation in trifluoromethanesulfonic acid conditions.9
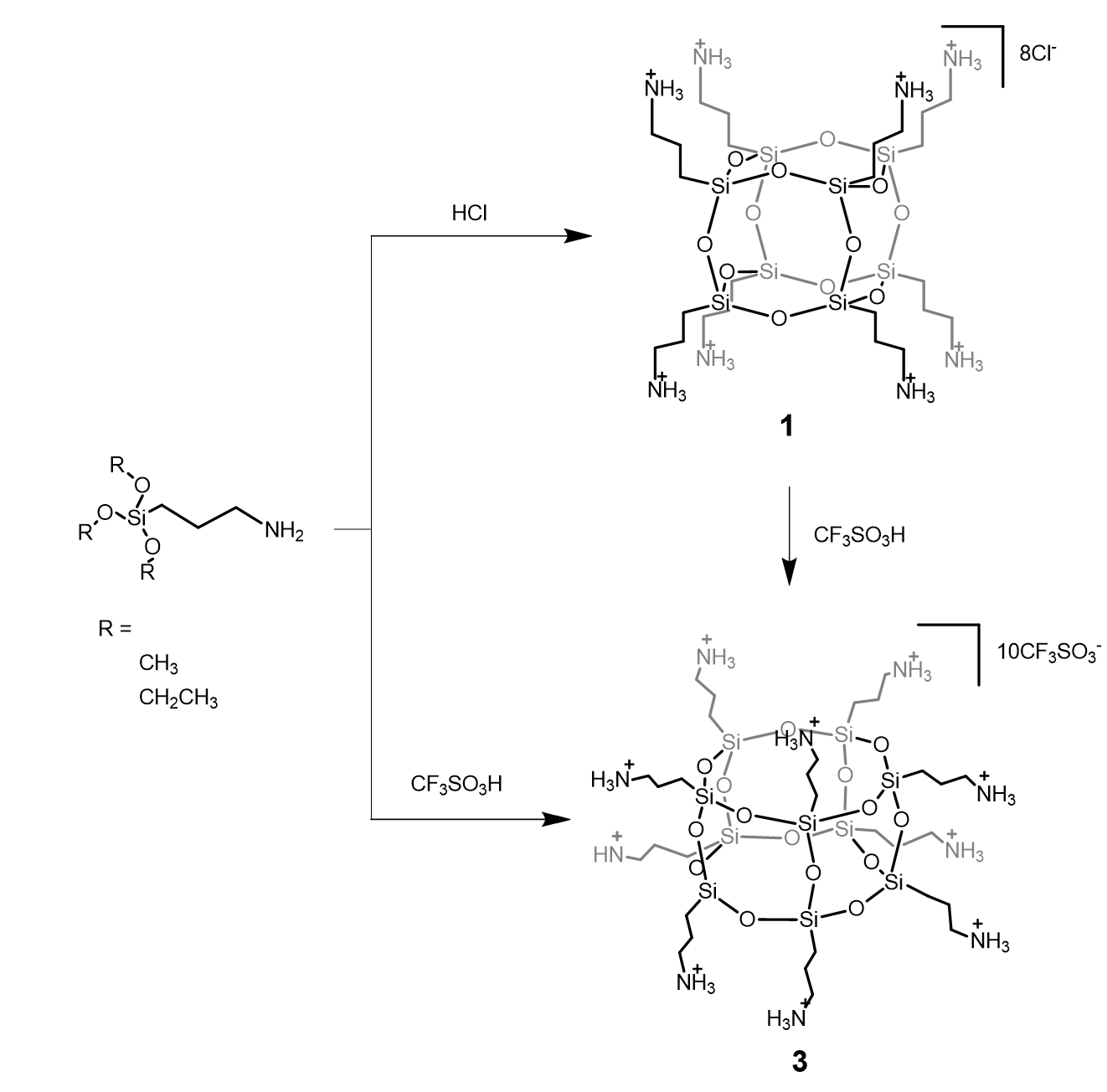
Direct synthesis of T10 POSS (3) from T8 (1) using superacid CF3SO3H (RSC Advances, 2015, 5, 72340-72351)
General methods for obtaining T10 and T12 cages are shown in scheme belowe. It is usually difficult to obtain phase clean T10 or T12 cages. The separation of POSS is particularly demanding when the compounds, for example T10 and T12, have similar hysicochemical properties, such as retention factor or similar solubility. The known T10-type silsesquioxanes were separated from the reaction mixture by means of fractional crystallization, sublimation, or using HPLC or SEC chromatography.

Methods of obtaining cage silsesquioxanes of T10 type
R. Laine et al. described the crystal structure of decaphenyldecasilsesquioxane.10 These studies allowed for unequivocal determination of the structure of the silsesquioxane core containing 10 silicon atoms. As shown in figure below, it has an idealized D5h symmetry.
Compounds of the T10R10 type typically are air-stable white powders. Their physical properties are similar to T8R8. The reactivity of T10-type silsesquioxanes is less well understood than that of their T8 analogs, but is usually similar. T. Goodson and R. Laine investigated the spectroscopic properties of T8, T10 and T12 derivatives containing a stilbene moiety.11 It turned out that with increasing the number of chromophore groups per silsesquioxane cage, the quantum luminescence yield decreased. It is related to self-absorption leading to non-radiative quenching. However, of the three frames (T8, T10 and T12), POSS T10 showed the highest two-photon absorption, indicating strong electron coupling and polarization.

X-Ray structure of decaphenylsilsesquioxane
(1) Frye, C. L.; Collins, W. T. Oligomeric Silsesquioxanes, (HSiO3/2)n. J. Am. Chem. Soc. 1970, 92, 5586–5588.
(2) Kawakami, Y.; Yamaguchi, K.; Yokozawa, T.; Serizawa, T.; Hasegawa, M.; Kabe, Y. Higher Polyhedral Silsesquioxane (POSS) Cage by Amine-Catalyzed Condensation of Silanols and Related Siloxanes. Chemistry Letters 2007, 36, 792–793.
(3) Bassindale, A. R.; Liu, Z.; MacKinnon, I. A.; Taylor, P. G.; Yang, Y.; Light, M. E.; Horton, P. N.; Hursthouse, M. B. A Higher Yielding Route for T 8 Silsesquioxane Cages and X-Ray Crystal Structures of Some Novel Spherosilicates. Dalton transactions 2003, No. 14, 2945–2949.
(4) Ervithayasuporn, V.; Wang, X.; Kawakami, Y. Synthesis and Characterization of Highly Pure Azido-Functionalized Polyhedral Oligomeric Silsesquioxanes (POSS). Chem. Commun. 2009, No. 34, 5130–5132.
(5) Ervithayasuporn, V.; Chimjarn, S. Synthesis and Isolation of Methacrylate- and Acrylate-Functionalized Polyhedral Oligomeric Silsesquioxanes (T8, T10, and T12) and Characterization of the Relationship between Their Chemical Structures and Physical Properties. Inorg. Chem. 2013, 52, 13108–13112.
(6) Chimjarn, S.; Kunthom, R.; Chancharone, P.; Sodkhomkhum, R.; Sangtrirutnugul, P.; Ervithayasuporn, V. Synthesis of Aromatic Functionalized Cage-Rearranged Silsesquioxanes (T8, T10, and T12) via Nucleophilic Substitution Reactions. Dalton Trans. 2015, 44, 916–919.
(7) Jaroentomeechai, T.; Yingsukkamol, P.; Phurat, C.; Somsook, E.; Osotchan, T.; Ervithayasuporn, V. Synthesis and Reactivity of Nitrogen Nucleophiles-Induced Cage-Rearrangement Silsesquioxanes. Inorg. Chem. 2012, 51, 12266–12272.
(8) Rikowski, E.; Marsmann, H. C. Cage-Rearrangement of Silsesquioxanes. Polyhedron 1997, 16, 3357–3361.
(9) Janeta, M.; John, Ł.; Ejfler, J.; Szafert, S. Novel Organic-Inorganic Hybrids Based on T8 and T10 Silsesquioxanes: Synthesis, Cage-Rearrangement and Properties RSC Advances, 2015, 5, 72340-72351.
(10) Roll, M. F.; Kampf, J. W.; Kim, Y.; Yi, E.; Laine, R. M. Nano Building Blocks via Iodination of [PhSiO1.5]n, Forming [p-I-C6H4SiO1.5]n (n = 8, 10, 12), and a New Route to High-Surface-Area, Thermally Stable, Microporous Materials via Thermal Elimination of I2. J. Am. Chem. Soc. 2010, 132, 10171–10183.
(11) Furgal, J. C.; Jung, J. H.; Goodson, T.; Laine, R. M. Analyzing Structure–Photophysical Property Relationships for Isolated T8, T10, and T12 Stilbenevinylsilsesquioxanes. J. Am. Chem. Soc. 2013, 135, 12259–12269.
Comments
Post a Comment