History of silsesquioxanes
Research on compounds containing Si–O bond for years has been dominated by silicon oxide, minerals containing the repeating fragment of SiO2 and silicones made of repeating units of R2SiO (R = alkane or phenyl). Over the past 20–30 years, research has been developing on silsesquioxanes containing the RSiO1.5 group. Due to the presence of an inorganic fragment and an organic group, these compounds have hybrid properties. The inorganic Si–O–Si fragment gives these compounds chemical and thermal resistance, while the organic R group increases their solubility and gives them appropriate reactivity. It is possible to obtain many polymeric structures with the general formula (RSiO1.5)n, but the most interesting are those with polyhedron structure.1
The first reports of compounds composed of repeating CH3SiO1.5 groups appeared in 1946 in the work of D. Scott.2 Scott described the thermal depolymerization of polymethylsiloxane obtained during the condensation of trichloromethylsilane with dichlorodimethylsilane. This reaction resulted in a white solid identified as octamethylsilsesquioxane with impurities of other polycyclic methylsilsesquioxanes, which was not confirmed by structural analysis. Subsequent studies concerned the identification of the compounds formed in these reactions. In 1954 A. Barry,3 as a result of tests of the hydrolysis reaction and subsequent condensation of silanes substituted with organic groups, synthesized compounds analogous to those obtained by D. Scott. They contained alkyl groups or a benzene ring as organic groups. Using the powder X-ray diffraction method, he determined the crystallinity of the resulting products, which was supposed to exclude the formation of the polymer. The presence of oligomeric compounds was found on the basis of a thorough analysis of the obtained experimental data, the ratio of silicon to organic groups was estimated by elemental analysis, the molar mass was determined cryometric, while the formation of closed cyclic forms, not containing the silanol group, was confirmed by infrared spectroscopy methods.3
Later research by J. Brown was based on obtaining polyhedral silsesquioxanes of various sizes of silsesquioxane cage.4,5 In the synthesis, J. Brown used the hydrolysis and condensation reaction of trimethylethoxysilane, and as a catalyst he used a solution of potassium hydroxide in a mixture of water and methanol. In the obtained product after crystallization, there was a mixture containing cages with different core sizes, ie T8, T10 and T12. Moreover, the structural structure of the particles obtained was proposed, which indicated the formation of polyhedrons. Using similar reaction conditions for the hydrolysis of trichlorophenylsilane, J. Brown obtained a mixture of phenylsilsesquioxanes containing 8, 10 and 12 silicon atoms.5 In 1990, the thermal properties of polyhedral silsesquioxanes were investigated for the first time. It turned out that these compounds have high thermal resistance, significantly exceeding the polymeric compounds obtained with the sol-gel technique.6 This observation contributed to the wider use of silsesquioxanes as additives to polymers, increasing their thermal resistance. In the 1990s, research on silsesquioxanes focused mainly on the development of methods for the synthesis of silsesquioxanes containing reactive groups as side arms and their functionalization. Currently, research is focused on the functionalisation of POSS molecules with previously unknown properties.
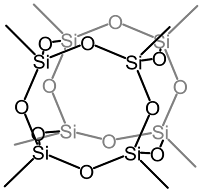
Structure of octamethyloctasilsesquioxane (Me8T8)
(1) Cordes, D. B.; Lickiss, P. D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173.
(2) Scott, D. W. Thermal Rearrangement of Branched-Chain Methylpolysiloxanes. J. Am. Chem. Soc. 1946, 68, 356–358.
(3) Barry, A. J.; Daudt, W. H.; Domicone, J. J.; Gilkey, J. W. Crystalline Organosilsesquioxanes . J. Am. Chem. Soc. 1955, 77, 4248–4252.
(4) Vogt, L. H.; Brown, J. F. Crystalline Methylsilsesquioxanes. Inorg. Chem. 1963, 2, 189–192.
(5) Brown, J. F.; Vogt, L. H.; Prescott, P. I. Preparation and Characterization of the Lower Equilibrated Phenylsilsesquioxanes. J. Am. Chem. Soc. 1964, 86, 1120–1125.
(6) Laine, R. M.; Rahn, J. A.; Youngdahl, K. A.; Babonneau, F.; Hoppe, M. L.; Zhang, Z. F.; Harrod, J. F. Synthesis and High Temperature Chemistry of Methylsilsesquioxane Polymers Produced by Titanium-Catalyzed Redistribution of Methylhydridooligo- and -Polysiloxanes. Chem. Mater. 1990, 2, 464–472.
Comments
Post a Comment