Octavinyloctasilsesquioxane (POSS-Octavinyl substituted) and its derivatives
Octavinyloctasilsesquioxane was first prepared in 1978 with a yield of <10% by K. Andrianov1 and co-workers in the trichlorovinylsilane hydrolysis reaction. Since then, many groups have tried to improve the method of obtaining it.2-4 In 1997, P. Harrison and Ch. Hall, when examining the hydrolysis of CH2=CHSiCl3 in ethanol, they obtained (CH2=CHSiO1.5)8 with an efficiency of 30%.5 The use of an ion exchange resin as a catalyst for the hydrolysis and condensation of CH2=CHSiCl3 contributed to an increase in the reaction efficiency to 40%.6 Compound (CH2=CHSiO1.5)8 was obtained with a yield of 80% using CH2=CHSi(OEt)3 during hydrolysis and tetramethylammonium hydroxide as a phase transfer reagent.7
Octavinyloctaasilsesquioxane derivatives can be obtained as a result of typical alkene reactions, such as: thiolation, phosphination, hydrosilylation, epoxidation, as shown in scheme below. Thiolation with reagents such as thiophenol or thiocyclohexane can be performed using an AIBN radical initiator or by exposing the system to UV radiation.8–11 The radical addition of phosphines and phosphonates, such as diethylphosphine or diethyl phosphate, to octavinyloctasilsesquioxane leads to the formation of functionalized silsesquioxanes containing phosphorus atoms attached to the silsesquioxane side arms (scheme below). These compounds have been used as coordinating ligands for transition metals, e.g. rhodium.12 Oxidation of the vinyl octavinyloctasilsesquioxane group with m-chloroperbenzoic acid (m-CPBA), transforms it into an epoxy. This moiety when used as a Lewis acid initiator undergoes a polymerization reaction to form an organic/inorganic composite.

Octavinyloctasilsesquioxane reactivity (AIBN: azobis (isobutyronitrile), m-CPBA: m-chloroperbenzoic acid)
The products of the hydrosilylation reaction catalyzed by platinum compounds between octavinyloctaasilsesquioxane and di- and trichlorosilanes are compounds that form a building block for the preparation of dendrimers. Upon reaction with Grignard reagent, POSS-dendrimer can be formed, containing 24 vinyl side groups per cubic silsesquioxane core (scheme below).
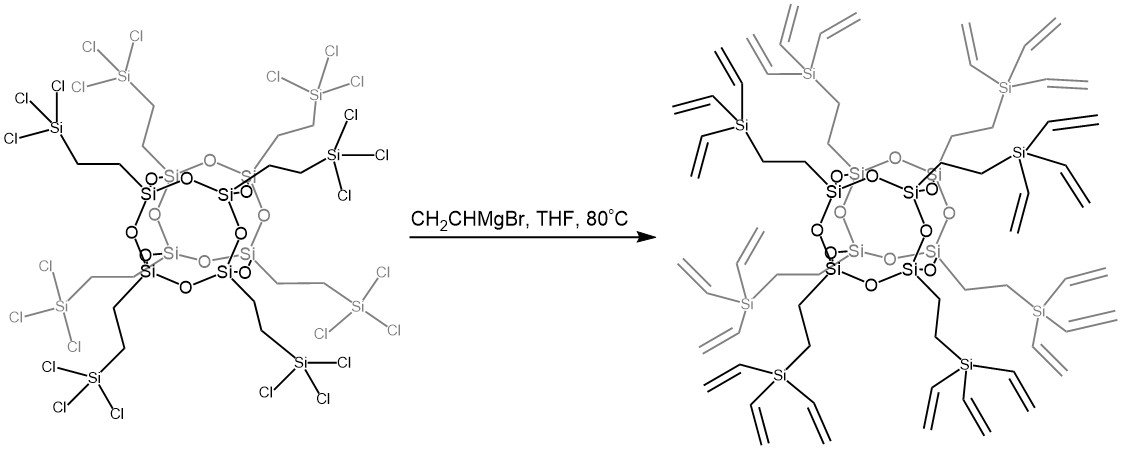
synthesis of dendrimers based on caged vinylsilsesquioxane
(2) Voronkov, M. G.; Martynova, T. N.; Mirskov, R. G.; Belyi, V. I. Octavinylsilsesquioxane. Russian Journal of Organic Chemistry 1979, 49, 1522–1525.
(3) Kovrigin, V. M.; Lavrent’ev, V. I. Chromatographic-Mass-Spectroscopic Study of the Mechanism of Fomation of Pervinyloctasilsesquioxane in Polycondensation of Vinyltrichlorosilane in Butanol. Russian Journal of Organic Chemistry 1989, 59, 377–383.
(4) Bonhomme, C.; Tolédano, P.; Maquet, J.; Livage, J.; Bonhomme-Coury, L. Studies of Octameric Vinylsilasesquioxane by Carbon-13 Andsilicon-29cross Polarization Magic Angle Spinning and Inversion Recovery Crosspolarization Nuclear Magnetic Resonance Spectroscopy. J. Chem. Soc., Dalton Trans. 1997, No. 9, 1617–1626.
(5) Harrison, P. G.; Hall, C. preparation and characterization of octasilsesquioxane cage monomers: Main Group Metal Chemistry. 1997, 20, 515–529.
(6) Dare, E. O.; Liu, L.-K.; Peng, J. Modified Procedure for Improved Synthesis of Some Octameric Silsesquioxanes via Hydrolytic Polycondenzation in the Presence of Amberlite Ion-Exchange Resins. Dalton Trans. 2006, No. 30, 3668–3671.
(7) Gao, J.; Wang, S.; Zhang, X.; Run, M. Synthesis of Cage Octa(Vinyl)Silsesquioxane. Youjigui Cailiao 2005, 19, 5–7.
(8) König, H. J.; Marsmann, H. C.; Letzel, M. C. Thioether Functionalized Octasilsesquioxanes. In Organosilicon Chemistry V; Auner, N., Weis, J., Eds.; Wiley-VCH Verlag GmbH, 2003; pp 425–428. (9) Gao, Y.; Eguchi, A.; Kakehi, K.; Lee, Y. C. Efficient Preparation of Glycoclusters from Silsesquioxanes. Org. Lett. 2004, 6, 3457–3460.
(10) Lücke, S.; Stoppek-Langner, K.; Kuchinke, J.; Krebs, B. Octakis-(Dimethylphosphanoethyl)-Octasilsesquioxane–Synthesis, Characterization and Reactivity. Journal of Organometallic Chemistry 1999, 584, 11–15.
(11) Piorecka, K.; Radzikowska, E.; Kurjata, J.; Rozga-Wijas, K.; Stanczyk, W. A.; Wielgus, E. Synthesis of the First POSS Cage–Anthracycline Conjugates via Amide Bonds. New J. Chem. 2016, 40, 5997–6000.
(12) Ropartz, L.; Morris, R. E.; Schwarz, G. P.; Foster, D. F.; Cole-Hamilton, D. J. Dendrimer-Bound Tertiary Phosphines for Alkene Hydroformylation. Inorganic Chemistry Communications 2000, 3, 714–717.
Comments
Post a Comment