Electronic properties of octameric silsesquioxanes
Quantum-mechanical calculations of octhydrooctasilsesquioxane showed that the highest occupied molecular orbital (HOMO) of this compound consists of atomic orbitals of free electron pairs of oxygen atoms, while the lowest energetically unfilled molecular orbital (LUMO) The lowest unoccupied molecular orbital) is spherical and is located in the center of the silsesquioxane core as shown in figure belowe. The calculations also showed that the energy gap between the HOMO and LUMO orbitals is approximately 6-7 eV. This value is higher than the limit for conductivity (3 eV), which proves that the silsesquioxane core is an insulator.

Figure of molecular orbitals: (a) HOMO and (b) LUMO octhydrocarbonoctasilsesquioxane
Taking into account the low electronegativity of silicon atoms (1.90 compared with 2.55 for the carbon atom according to the Pauling scale), it can be assumed that the POSS core will behave like an electron donor group. However, experimental studies have shown that the silsesquioxane core behaves as an electron withdrawing group. F. Feher and T. Budzichowski proved that the 4-(chloromethyl) phenyl group attached to silsesquioxane is not susceptible to hydrolysis and does not undergo a substitution reaction. Based on the analysis of chemical shifts on the 13C NMR spectra of octameric silsesquioxanes, they determined that the electron-accepting properties of silsesquioxane are comparable to the trifluoromethyl group (-CF3).
Another evidence of the electrophilic properties of the silsesquioxane core is the possibility of trapping the fluoride anion inside the silsesquioxane core. Obtaining such a system is possible thanks to the use of n-butylammonium fluoride during the condensation reaction of triethoxysilane or in the reaction of tetramethylammonium fluoride with octameric silsesquioxane. these can be isolated only when the organic side groups are weakly electron-accepting, such as phenyl, vinyl or fluorinated alkyl groups.
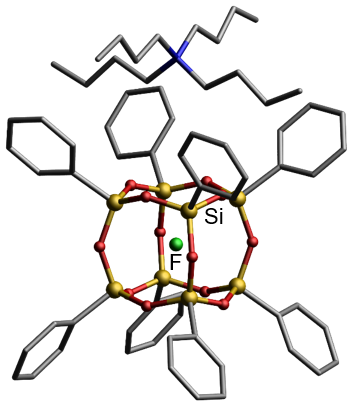
X-ray structure in the solid octasilsesquioxane with the trapped fluoride anion inside the POSS core (hydrogen atoms omitted)
(1) Pauling, L. The Nature of the Chemical Bond. IV. The Energy of Single Bonds and the Relative Electronegativity of Atoms. J. Am. Chem. Soc. 1932, 54, 3570–3582.
(2) Feher, F. J.; Budzichowski, T. A. Syntheses of Highly-Functionalized Polyhedral Oligosilsesquioxanes. Journal of Organometallic Chemistry 1989, 379, 33–40.
(3) Bassindale, A. R.; Pourny, M.; Taylor, P. G.; Hursthouse, M. B.; Light, M. E. Fluoride-Ion Encapsulation within a Silsesquioxane Cage. Angew. Chem. Int. Ed. 2003, 42, 3488–3490.
(4) Bassindale, A. R.; Parker, D. J.; Pourny, M.; Taylor, P. G.; Horton, P. N.; Hursthouse, M. B. Fluoride Ion Entrapment in Octasilsesquioxane Cages as Models for Ion Entrapment in Zeolites. Further Examples, X-Ray Crystal Structure Studies, and Investigations into How and Why They May Be Formed. Organometallics 2004, 23, 4400–4405.
(5) Anderson, S. E.; Bodzin, D. J.; Haddad, T. S.; Boatz, J. A.; Mabry, J. M.; Mitchell, C.; Bowers, M. T. Structural Investigation of Encapsulated Fluoride in Polyhedral Oligomeric Silsesquioxane Cages Using Ion Mobility Mass Spectrometry and Molecular Mechanics.”. Chem. Mater. 2008, 20, 4299–4309.
Comments
Post a Comment